What Determines the Color of Something?
Important update: These pages have moved to their own domain at https://rainbowspec.observer, where their information, organization, and graphics have been significantly improved! This page is replaced by my new color page.
Pigments and Light
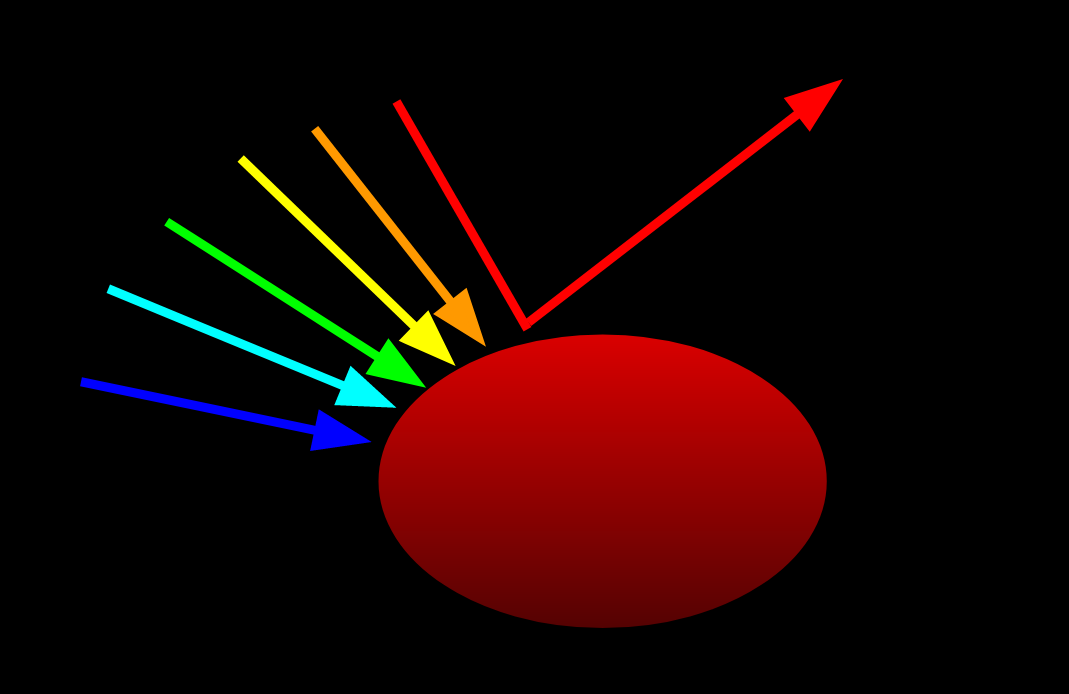
Pigments are not unrelated to light. When white light hits an object, said object absorbs some wavelengths and reflects others. The reflected wavelengths determine the color of the object since those are the ones that hit our eyes. A red object absorbs all wavelengths except red. A purple object absorbs all wavelengths except red and blue. A white object reflects all wavelengths of light. A black object absorbs all wavelengths of light. Now not all of the light is absorbed/reflected, otherwise white objects would blind us, and black objects would appear to have no dimension. It is more the ratio of the different wavelengths (colors) that are absorbed/reflected that determine the color.
So what determines what color something is? Why are bananas yellow/green instead of red? There are a few different theories on the chemical causes of color, but all involve the specific amount of energy an atom, compound, or crystal structure needs to reach a higher energy state.Causes of Color They are the most stable at ground state, their lowest energy state, but they have higher energy states they can be at. These states are at very specific energies which are different for each molecule.
Light is a form of energy, thus it can give said energy to the molecules. Most importantly is the fact that these molecules can only reach a higher energy state if the energy it receives is exactly the same as the difference between a higher energy state and the initial. Each color of light has a different amount of energy. If the energy of a color of light matches that of the energy needed to excite the molecule to higher energy state(s), said molecule will absorb that/those color(s). The rest of the colors will be reflected off of the molecule. Atoms and Light
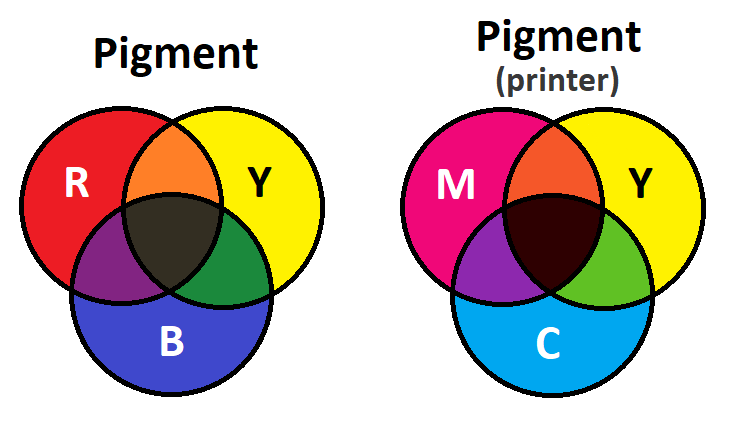
Pigments and dyes follow the subtractive color model, which means at each additional pigment, the darker the resultant color gets. Ideally an equal mix of primary colors will create black. The more transparent the material is, the truer to reality this is. For that reason, transparent dyes can approximate black better than opaque pigments.
The reason a mixture of pigments results in less color is because the different chemical structures that absorb wavelengths are layered, meaning more light is absorbed, and less is reflected. Consider blue and orange paint mixed together. The chemical structures making blue and the ones making orange are still intact and distinct, simply in tight proximity. Thus, while the blue paint would normally reflect the blue wavelengths, the orange pigment absorbs some of them, likewise the blue pigment absorbs some of the orange wavelengths normally reflected by the orange, resulting in less light overall being reflected. Whether this mixture results in brown or a warm dull blue depends on the ratio of the colors.