The Science of Rainbows - Introduction
Important update: These pages have moved to their own domain at https://rainbowspec.observer, where their information, organization, and graphics have been significantly improved! This page is replaced by the new homepage and a new page dedicated to the visible spectrum.
These pages are my notes on how rainbows and related phenomenon occur. Note that this is a passion project made by an amateur; there may be typos and some information may not be accurate due to flaws in my understanding. Information is credited on the sidebar under "Sources" or "Related Sites". Basic information found in any physics textbook is not credited.
These pages use JavaScript for light/dark mode buttons and to keep the page navigation consistent for all pages.
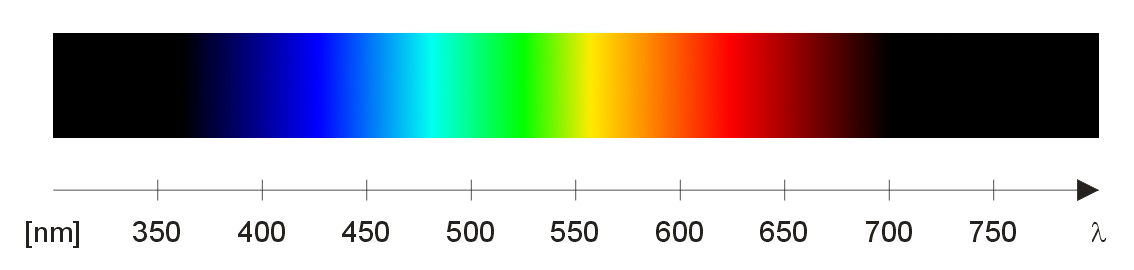
The Visible Spectrum
The visible spectrum is a portion of the electromagnetic spectrum. The electromagnetic spectrum organizes particles of energy by their wavelengths and amount of energy. These particles of energy are called photons. Photons travel at the speed of light in waves. The amount of energy these photons have determines their properties (hence the separation of the electromagnetic spectrum into categories). Light and heat are forms of energy (the visible spectrum and infrared radiation). X-rays, microwaves, UV-rays, and radio waves are other forms of energy made of photons.
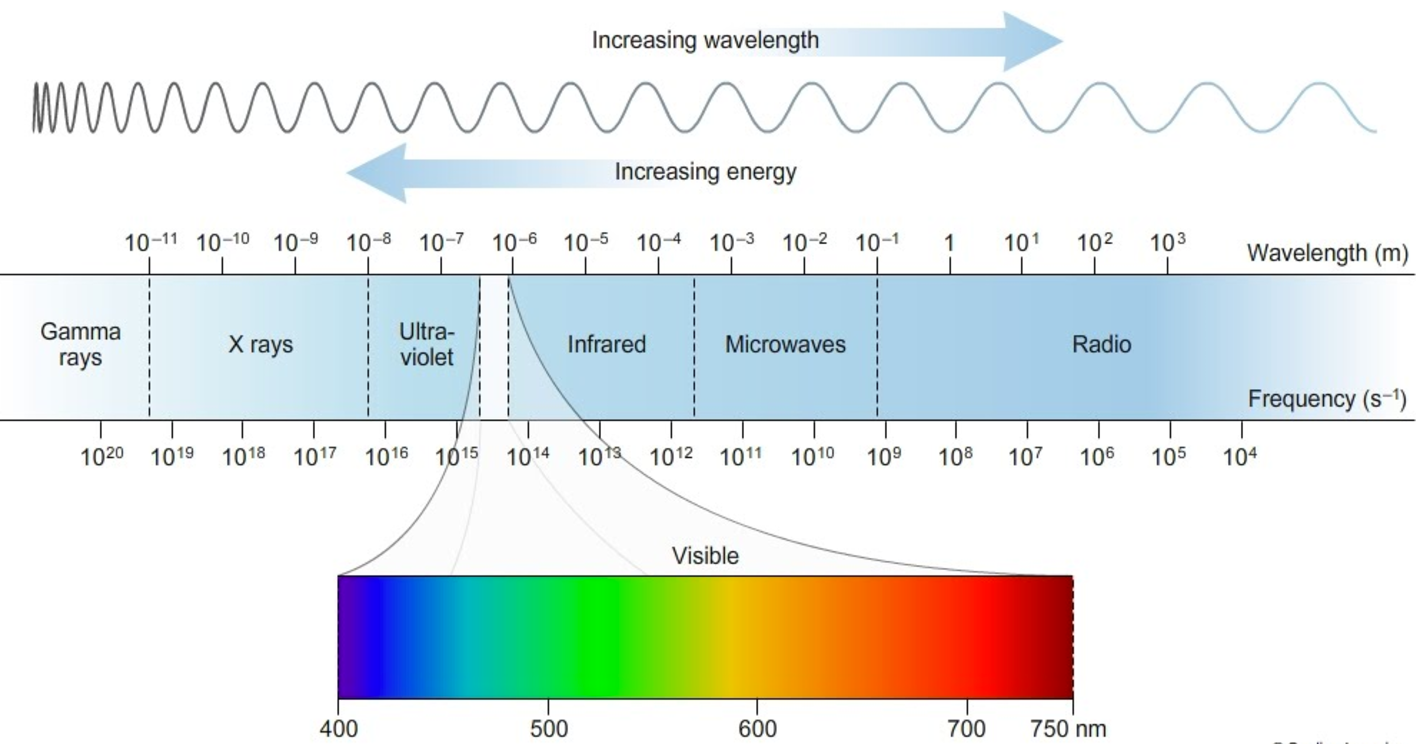
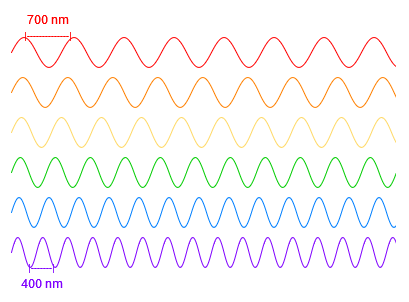
Because they all travel at the same speed, the shorter the wavelength, the higher the frequency of waves, thus the higher the energy.
The photons (energy) in the visible spectrum take the form of light. Infrared radiation is heat (radiation = emission of energy). Visible light, infrared radiation, microwaves, and radio waves are forms of nonharmful radiation, as the photons do not have enough energy to break chemical bonds or ionize molecules. UV radiation, X-rays, and Gamma rays are harmful to living cells because the photons do have enough energy to cause chemical reactions. This is why you wear a lead cover when getting a dental X-ray, as X-rays can't pass through lead. The sun emits UV radiation, which is why sunscreen is important.

Each wavelength (380-750 nanometers) corresponds to a different color. Violet wavelengths are the shortest and red are the longest.
Purple and the Visible Spectrum
Notice how the visible spectrum spans from purplish blue to red, but light can come in more colors (magenta, purple, white, etc).
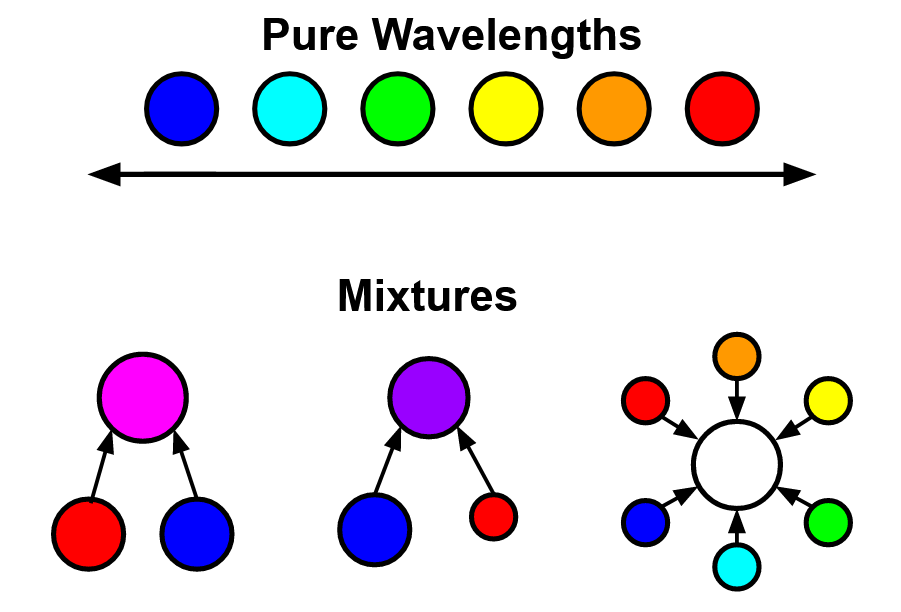
This is because colors like magenta, purple, and white are made by different wavelengths of light mixed together. White light is an even mixture of all wavelengths, magenta is an equal mixture of blue and red, and purple is also a blend of blue and red, but with a higher ratio of blue. In short, white, pink, and purple do not have their own wavelengths.
If you search online for the visible spectrum you will get many sources including purple in the visible spectrum. This is not necessarily incorrect, but it can be misleading considering purple is a wide color category. The color purple includes both colors that are on the short end of the visable spectrum, and colors that can only be made by mixing wavelengths.
Violet is often used to refer to the blue-er end of purple colors, which have their own wavelength in the visible spectrum. To visualize the color of the smallest visible wavelength, think of the blue-ish purple light emitted by a black light. A black light emits mainly UV radiationnote and a little bit of visible light on the edge of UVn which is violet. This blue-ish purple is where the short end of the visable spectrum ends. Any warmer purples are created by mixing red wavelengths with blue or violet wavelengths.
note: UV radiation is harmful, but black lights are weak and typically only comprised of the less harmful long UV waves close to visible light.